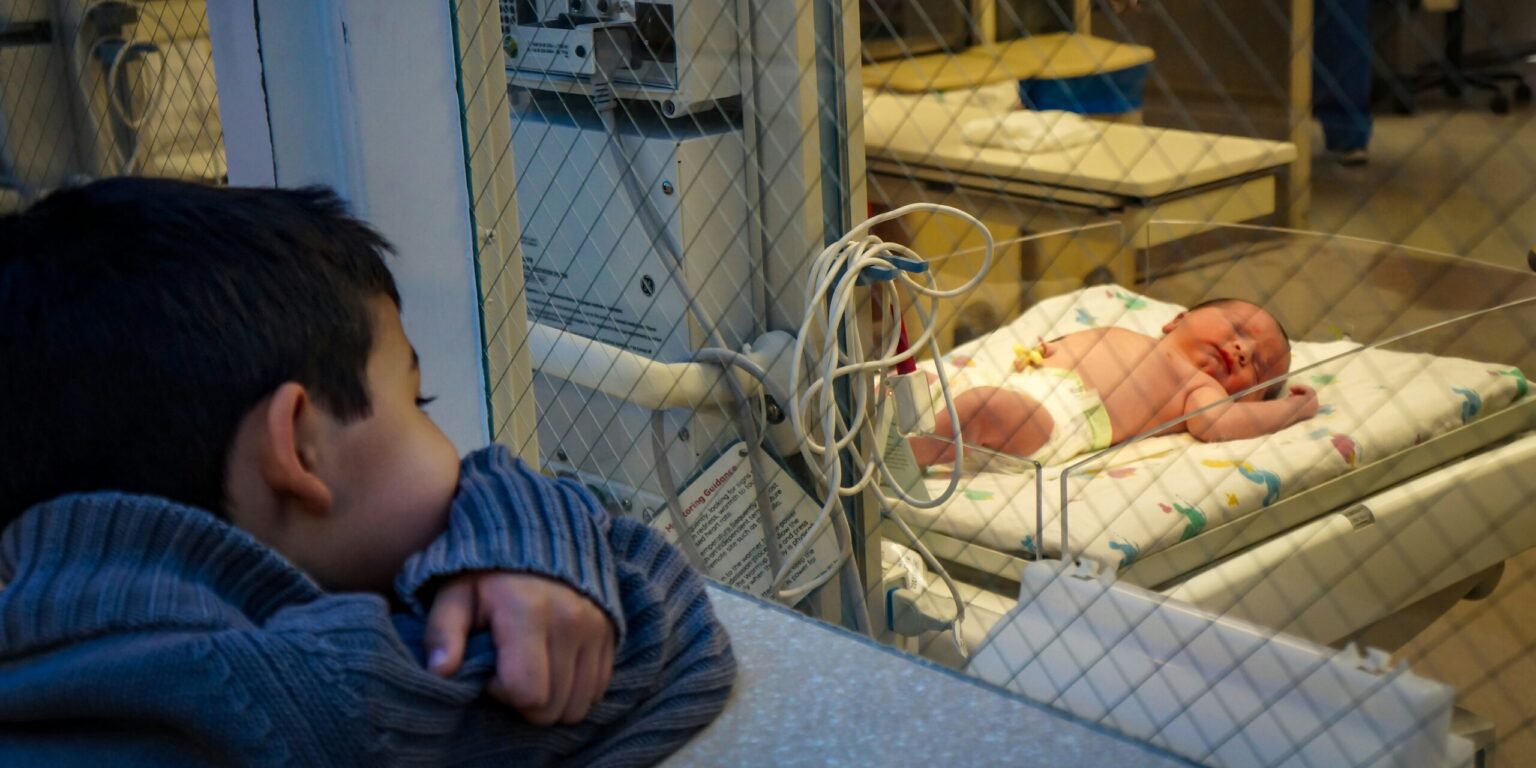
In an unprecedented achievement for the field of genetic medicine, doctors in the United States have successfully treated a newborn baby using a personalized CRISPR-based gene-editing therapy. The treatment, developed in record time, targeted a rare and previously incurable genetic condition and marks the first known use of a bespoke gene-editing therapy tailored specifically for a single patient. The case is being hailed as a potential breakthrough that could reshape the future of how rare genetic disorders are managed and treated.
The infant, named KJ Muldoon, was diagnosed shortly after birth with carbamoyl phosphate synthetase 1 (CPS1) deficiency, a life-threatening urea cycle disorder that impairs the body’s ability to eliminate ammonia from the bloodstream. Without treatment, this condition can result in irreversible brain damage, coma, or death within days or weeks. CPS1 deficiency is exceedingly rare, affecting approximately one in a million newborns globally.
Traditional treatment options for CPS1 deficiency are limited, typically involving lifelong medication, dietary restrictions, and in some cases, liver transplantation. In KJ’s case, doctors and geneticists at the Children’s Hospital of Philadelphia, in collaboration with Penn Medicine and the Innovative Genomics Institute, opted to pursue an experimental path. They designed a custom CRISPR base-editing therapy that would correct the genetic mutation responsible for the disorder directly in KJ’s liver cells. The therapy was delivered through lipid nanoparticles—tiny fat-like carriers that transported the gene-editing molecules to the liver, where CPS1 is normally produced.
Unlike traditional CRISPR techniques that cut DNA strands, this new base-editing method chemically altered a single letter of KJ’s genetic code without breaking the DNA. This approach significantly reduces the risk of unintended mutations and off-target effects, which are among the primary concerns in gene-editing procedures. The entire process—from diagnosis to delivery of the therapy—was completed in under six months, a timeline that researchers say was made possible by years of foundational research and a highly coordinated multi-institutional effort.
The first dose was administered in February 2025, when KJ was just a few weeks old. Two more doses followed in March and April. By May, doctors began observing promising results. KJ was able to tolerate higher levels of protein in his diet, a key marker of liver function improvement. He also required fewer medications to manage ammonia levels in his blood, and most notably, he began meeting key developmental milestones that children with untreated CPS1 deficiency typically miss. Now more than nine months old, KJ is thriving, showing signs of typical growth and cognitive development, and weathering routine illnesses that would otherwise pose severe threats to a child with his condition.
Medical professionals involved in the case describe it as a proof of concept for individualized gene-editing therapies. The successful outcome suggests that, with the right genetic target and delivery system, it is possible to design treatments on a case-by-case basis for patients suffering from rare diseases that lack conventional therapies. While this therapy was highly specific to KJ’s particular mutation and disorder, scientists believe it could be a model for how rare genetic diseases might be approached in the future.
The cost of the therapy—around $800,000—was roughly equivalent to that of a liver transplant, and researchers note that prices could come down if similar treatments are scaled up and standardized. Importantly, the therapy was administered under strict regulatory oversight, and because the genetic changes were limited to somatic cells (in this case, liver cells), they are not passed on to future generations. This distinction separates the procedure from controversial germline editing, which alters the DNA of embryos and has been widely condemned due to ethical concerns.
Ethicists and medical experts have praised the team for adhering to rigorous safety and ethical standards. KJ’s parents, Kyle and Nicole Muldoon, made the difficult decision to proceed with the experimental therapy after extensive discussions with doctors. They considered other options, including liver transplantation, but ultimately chose the gene-editing route in hopes of offering their son a better quality of life. Their courage and willingness to participate in the trial have provided invaluable data that could guide similar treatments for other children in the future.
The broader implications of this case are significant. It comes at a time when gene-editing technologies like CRISPR are gaining traction in treating blood disorders, cancers, and hereditary diseases. Previous successes, such as the approval of CRISPR-based therapies for sickle cell disease and beta thalassemia, have already demonstrated the potential of genome editing. However, those treatments typically involve editing stem cells outside the body and reinfusing them, a far more complex and invasive process. In contrast, the success in KJ’s case—where editing occurred directly inside the body—is seen as a leap forward in making genetic therapies more accessible and less invasive.
Still, experts caution that this is just the beginning. Long-term monitoring will be required to determine how durable the benefits are and whether any delayed side effects emerge. Larger clinical trials will be necessary to confirm the safety and effectiveness of personalized therapies across a more diverse patient population. But even with these caveats, the scientific community agrees that KJ’s treatment marks a milestone in medicine.
As research continues and regulatory frameworks evolve to support innovative therapies, KJ’s story could represent a pivotal moment in the transition from generalized care to precision medicine—where the treatment is not just for the disease, but for the individual.